- Home
- FAQ & Contact
FAQ & Contact
This is your source for quick information on the nuts and bolts of doing business with us. Here we have tried to answer the most common questions on everything from ordering to delivery, credits and recycling.
Use it as a look-up tool in your daily work or as a refresher when needed.
Contacts
| State | Customer Service | Phone |
|---|---|---|
| NSW | [email protected] | 1800 BAXTER / 1800 229 837 |
| QLD & NT | [email protected] | |
| SA | [email protected] | |
| VIC & TAS | [email protected] | |
| WA | [email protected] |
| State | Sales Specialist | Phone |
|---|---|---|
| NSW & ACT | MINA YOUSSEF | +61 (417) 412 226 |
| VIC, SA, NT & TAS | NICHOLAS EFTHIMIOU | + 61 (419) 555 048 |
| WA | BRENDON HILL | + 61 (400) 600 324 |
| QLD | KARA MOANA | +61 (408) 408 144 |
| NATIONAL | PAUL VALENTON | +61 (419) 988 589 |
| NATIONAL | PRISCILLA KURNIAWAN | +61 (419) 010 936 |
| NATIONAL | STEFAN KLJAJEVIC | + 61 (419) 003 349 |
FAQs
As soon as you become aware of an adverse event, which involves any of Baxter’s compounded goods, please contact your local customer service team or report the event online. We will ensure the adverse event is passed on promptly for further investigation.
Patient safety is our most important priority.
Report event to [email protected]
Baxter Compounding has several 3rd party closed system attachments that have been validated for use and can be attached in the compounding process.
A list of current validated attachments are as follows:
| Product Code | Description |
|---|---|
| 10012241 | Alaris Closed Male Luer (Texium) |
| 72951NE | Alaris Smart Site Needle Free System |
| MFX2302EV | Alaris with Filter |
| 120124XSK or CE120124XSK | Bodyguard Set |
| 120124XFSK or CE120124XSFK | Bodyguard Set with 1.2 Micron Filter |
| A2581NF | Cyto-Set Line without Safesite |
| A2903N | Cyto-Set Mix 0.22μm Filter |
| 011CL10 | ChemoLock Bag Spike |
| 011CL12 | ChemoLock Bag Spike with Additive Port |
| EMC1402 | Clearlink Spike |
| 764382 | Secondary Codan Connect Z Set |
| 764381 | Secondary Codan Connect Z Filter Set |
| SA-EZ | Equashield EZ Bag Spike Adaptor |
| FC-1 | Equashield Female Luer Lock Connector |
| SA-EZE | Equashield Set |
| SA 18050 | Equashield 180 Adaptor |
| 515306 | BD Phaseal Infusion Adaptor C100 |
| 515078 | BD Phaseal Optima Adaptor C100-0 |
| 515003 | BD Phaseal Injector Luer Lock N35 |
| 515052 | BD Phaseal Optima Injector Luer Lock N35-0 |
| 515302 | PhaSeal Secondary Set C61 |
| 515312 | BD Phaseal Secondary Set C62 |
| 7210.91 | Qimo Male Connector |
| CH2000SPC | Spiros (spinning) Connector with Purple Cap |
| 011CH10 | Clave Connector Bag Access Device |
| 011CH12 | Bag Spike with integrated Clave connector |
| 011CL3511 | Chemolock Set |
| 011CL200SPC | Chemolock Connector |
If you require further information, please get in touch with your Baxter contact on 1800 BAXTER (229 837) .
Baxter offers a comprehensive Clinical Trial Service for our Partnered Customers. This service commences with the receipt and storage of IMPs at our facilities, followed by the preparation and delivery of compounded doses to clients. Each stage of the process is meticulously validated, calibrated, and continuously monitored to ensure the highest standards of quality and compliance.
To accommodate diverse client needs, our service can be tailored to varying levels of involvement. Clients have the flexibility to choose the extent of our participation, including the maintenance of vial accountability logs. For further information on our Clinical Trial Service, please consult with your designated Compounding Sales Specialist.
You may have patients who are receiving compassionate drug vials. At Baxter, we can compound compassionate stock for you. If the compassionate stock is a drug and in a final presentation that we already provide, we can assign our Baxter extended shelf life.
If it’s a drug or presentation that we haven’t reviewed a Stability Agreement is required.
Explore Stability Tool >
Compounding Complexity
Compounding Considerations
Standard compounding
- Ordinary reconstitution of drug vials
- Dose volume draw up and transfer into final container
- Number of drug vials <10 for any typical dose
- Shelf life assigned from the manufacture time
- Typical compounding time is less than 20 to 30 minutes
- Examples of drugs include typical chemotherapies and monoclonal antibodies
Complex compounding
- Large number of drug vials per typical dose, such as 10 to 15
- Compounding time is greater than 20 to 30 minutes
- Two simple drugs compounded into one final container
- Examples include Bendamustine, Cyclophosphamide, Mitomycin, Paclitaxel Suspension, and Vedolizumab
Complexity 1
- Complex compounding steps requiring instructions to be followed
- Special vial reconstitution or dilution instructions, such as injecting drug into diluent vial
- Long compounding time such as 30 minutes or greater
- Shelf life of compounded product is short, such as 12 hours or less
- Examples include Sacituzumab Govitecan and SC Epcoritamab.
Complexity 2
- Complex compounding steps requiring instructions to be followed, recording of times, or activities requiring additional worksheets
- Special vial reconstitution or dilution instructions, such as injecting drug into diluent vial
- Long compounding time such as 45 minutes or greater
- Shelf life of compounded product is short, such as 8 hours or less
- Examples include Furosemide
Complexity 3
- Preparations or instructions outside of the cleanroom required, for example, special picking instructions, manual time records or time limits for packing
- Long compounding time such as 45 minutes or greater
- Shelf life of compounded product is very short, such as 4 hours or less
- Examples include compounding of targeted drug delivery such as DC beads
Complexity 4
- Complex compounding steps requiring instructions to be followed and/or instructions outside of the cleanroom required
- Compounding of blood and plasma products (Australia only)
- Long compounding time such as 60 minutes or greater
- Examples include compounding of Blinatumumab and Human IVIG
Fluid bag sizes and volumes
This information can be found in our stability tools accessible via mobile or PC. You can also download a pdf version.
Infusors and intermates
Download pdf for minimum and max allowable volume for infusors and intermates.
Your Compounding Sales Specialist can also conduct an infusor in-service with you. During the in-service your Sales Specialist can go through our infusor product range in detail, answer any questions, and provide additional HCP and patient resources including: The Instructions for Use and Patient Information.
Additional info: Elastomeric Professional Guide >
Infusors and intermates have specified minimum and maximum fill volumes. Can Baxter compound infusors with volumes outside these ranges?
Baxter Recognises that volumes outside the stated minimum and maximum may be necessary to account for changes in a solution viscosity or to adjust delivery time based on the prescription required. Health Care Professionals who choose to use fill volumes outside the range specified by the minimum and maximum should expect delivery variance compared to that specified in the Instructions for Use.
If you need a credit please contact Customer Service. We work as quickly as we can, but they usually take 1-2 weeks to process.
If you experience a faulty product, please call or email Baxter Compounding or your Sales Specialist. They will make sure your product complaint is investigated by our quality teams.
We ask that non-cytotoxic and unused faulty products are returned. As cytotoxic products and partially used product pose a safety hazard to handlers, we ask that photos of the sample, if possible, is forwarded instead.
Baxter Compounding operates under the provision of the Therapeutic Goods Regulations (TGA) 1990 as a contract manufacturer. This means a Good Manufacturing Practice (GMP) agreement must be signed and renewed every 5 years. This agreement is separate to your commercial agreement.
We will contact you with its time for renewal.
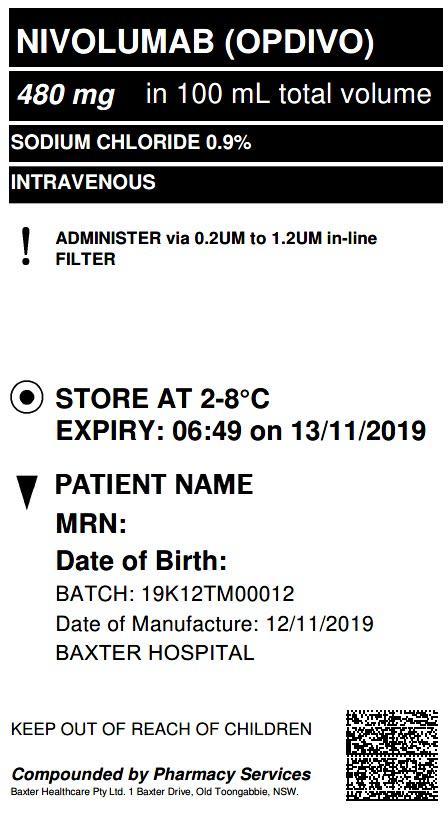
This is an example of what our labels look like.
Our packaging of cytotoxic drugs and monoclonal antibody immunotherapies helps identify the contents of each box, thereby making it clear and safe for staff when receiving and processing our products.
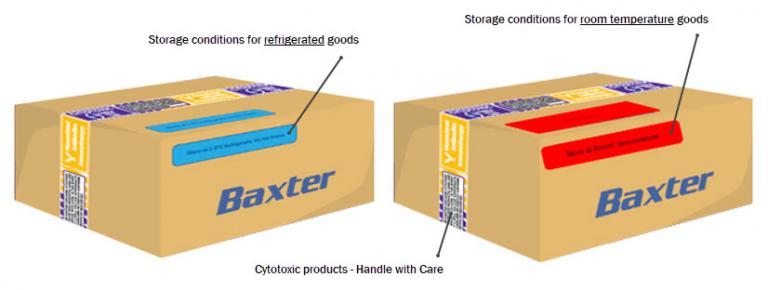
Over pouches
All products inside the boxes are sealed in over pouches:

|
= Cytotoxic products - purple and/or black over-pouches |

|
= Monoclonal antibody drugs - clear and/or black over-pouches |
Boxes are taped securely with Baxter Compounding tape to clearly identify compounded product.
Storage conditions are clearly marked with blue stickers to indicate refrigerated goods and red stickers to indicate room temperature goods.
Over pouches
All products inside the boxes are sealed in over pouches:

|
Antibiotic drugs – green over pouch |

|
Non-cytotoxic drugs – clear over pouch |
Baxter offers mg pricing for most drugs, however there are a few that are priced per vial.
For vial priced drugs, the total number of vials used is calculated per invoice. All drug doses ordered under one purchase order for one delivery date and time will be charged on the one invoice, and the number of vials used is calculated from this invoice.
If the same purchase order is used for the same delivery day but a different delivery times, the drug doses will be calculated on two separate invoices.
For our metro customers, gel bricks can be re-used, cardboard boxes and foil can be recycled rather than going to landfill. We do not accept cardboard boxes or foil back, however, reach out to your Sales Specialist to discuss what options are available for you and your hospital to recycle ice bricks and/or woolpacks.
Baxter Compounding has on file an SDS for all drugs compounded at Baxter. If you require a copy, please email your request to [email protected]
If you require a drug, a dosage or a final presentation that Baxter Compounding does not have stability for, the hospital pharmacy or clinician can complete a Stability Agreement (SA) form. This form allows you to assign shelf life and storage conditions for your requested product. Baxter Compounding will review the SA and supporting evidence, which will usually take 1-5 business days.
For any new drugs that Baxter has not reviewed, or for unregistered drugs, our team will need to assess the suitability of our services to compound the requested drug. The review usually takes 1-5 business days followed by implementation.
Requirements:
- Sterile drugs only
- Safety data sheet
- Product information and stability data must be provided
Please contact Baxter Compounding or your Sales Specialist for further information.
Tip
Did you know you can get Baxter stability data on your mobile?
Go to the Baxter Compounding app
Baxter’s shipping validation for room-temperature products is 40 hours and refrigerated products is 24 hours.
Specific drug information
The following table lists drugs that have special ordering requirements and labelling information. Please ensure you follow the ordering requirements for the applicable drugs below.
We support the use of biosimilars. Please note, that the order must specify the brand that you would like us to compound. If a brand is not specified Baxter will compound and invoice the originator brand.
All doses of Bleomycin must be ordered with the unit specified as IU
All purchase orders for brentuximab vedotin must be specified as mg of brentuximab vedotin. A purchase order must state “brentuximab vedotin X mg” where X mg is the dose of brentuximab vedotin (and not brentuximab base).
All purchase orders for eribulin mesilate must be specified as mg of eribulin mesilate. A purchase order must state “eribulin mesilate X mg” where X mg is the dose of eribulin mesilate (and not eribulin base).
All purchase orders for etoposide phosphate must be specified as mg of etoposide base. A purchase order must state “Etoposide (as phosphate) X mg” where X mg is the dose of etoposide base.
Baxter compounded etoposide phosphate products are labelled “ETOPOSIDE (AS Phosphate) X mg, where X is the number of milligrams of the etoposide base required.
All doses for Heparin must be ordered with the unit specified as IU
All doses for Insulin must be ordered with the unit specified as IU
Both 100mg and 900mg doses must be entered under the same order number and for the same delivery date and time such that the cost of one vial can be shared across both compounded doses.
Baxter compounded Piperacillin-Tazobactam products are labelled “PIPERACILLIN/TAZOBACTAM Xmg” where Xmg is the total dose of Piperacillin and Tazobactam sodium. For example, a dose of piperacillin 12g and tazobactam sodium 1.5g is labelled “PIPERACILLIN/TAZOBACTAM 13500mg”.